WHY OUR CELLS
QUALITY CONTROL PROGRAM
To ensure that the Stem cells we use in the therapies are safe and of the best quality, regardless of whether the source is Autologous (Bone marrow) or Allogenic (Ethically sourced umbilical cord mesenchimal stem cells) we design a 3-step quality control program, with specific analysis performed by authorized third-party laboratories.
OUR 3 STEP PROCESS
- 1stBefore stem cell isolation
- 2ndAfter isolation but before cell expansion
- 3rdDuring the culture process before the harvest
1st
BEFORE STEM CELL ISOLATION
Designed to evaluate the samples from where the stem cells will be obtained before processing them, in this analysis, its safety is verified through a complete microbiological profile, and a karyotype confirms its genetic quality by band staining GTW.
2nd
AFTER ISOLATION BUT BEFORE CELL EXPANSION
The second step is designed to verify that the isolated cells are mesenchymal stem cells, characterization is performed according to the International Society of Cell Therapy (ISCT). This includes morphology, expression of mesenchymal stem cell markers, no expression of hematopoietic stem cell markers, HLA type I, and potential for cell differentiation.
3rd
DURING THE CULTURE PROCESS BEFORE THE HARVEST
The last step is designed to verify the correct handling of the cells throughout the process; for this, another cell karyotyping is performed to demonstrate that no genetic errors were induced during the expansion; on the other hand, a microbiological analysis is carried out, that includes the main contaminants within hospitals and the product of human management. 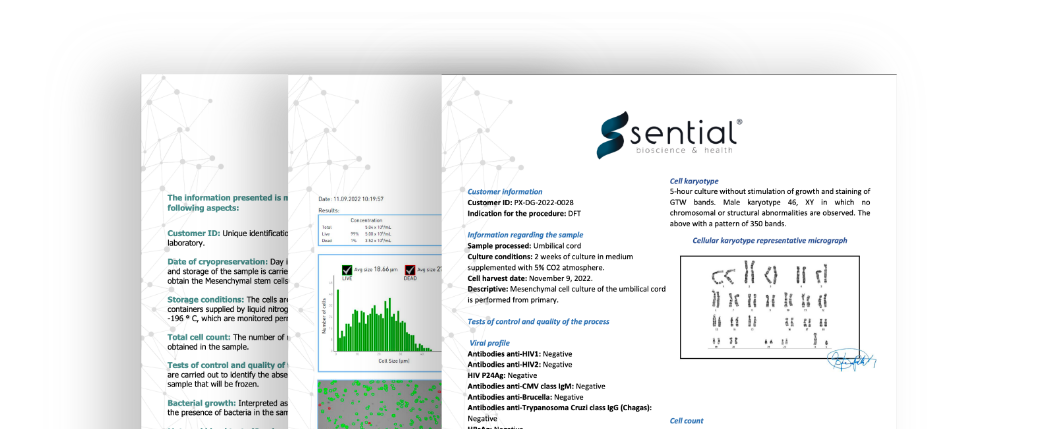
CERTIFICATE OF ANALISYS
After your therapy, you will receive a certificate of analysis of the product validated and signed by the Scientific Director, which includes:
- 1. Customer information regarding the sample.
- 2. Viral profile.
- 3. Cell karyotype.
- 4. Cell count.
- 5. Automated cell count report.
- 6. Calculation of the number of cells.
COLLABORATE
WITH US
ARE YOU A PHYSICIAN, CLINIC OR HOSPITAL INTERESTED IN OFFERING
STEM CELL THERAPIES?